- All-in-one solution for inclusion body protein isolation and purification
- Fast and convenient spin column protocol
- Complete kit with Cell Lysis Reagent, Inclusion Body Solubilization Reagent, buffers and spin columns to purify proteins
ProteoSpin™ Inclusion Body Protein Isolation Micro Kit
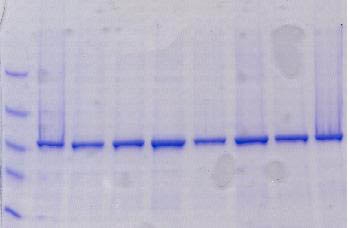
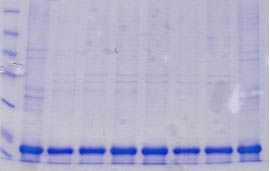
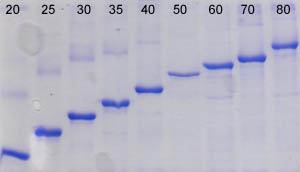
This kit provides everything required to isolate and purify inclusion body proteins from induced bacterial cultures. First a proprietary Cell Lysis Reagent is used to selectively lyse the cells and release inclusion bodies in their solid form. Next, inclusion bodies are dissolved and their contents released using the provided IB Solubilization Reagent. Inclusion body proteins are then further purified using spin columns for rapid and convenient buffer exchange and desalting. This kit provides a convenient way to screen recombinants prior to scaling up.
About Inclusion Bodies
Bacteria are widely used for the expression of different proteins. However, 70-80% of the proteins expressed in bacteria by recombinant techniques are typically contained in insoluble inclusion bodies (i.e., protein aggregates). The protein of interest found in these sub-cellular structures is often inactive, due to incorrect folding. The production rate of recombinant proteins stored in inclusion bodies is invariably higher than those synthesized as soluble proteins. The reason behind this is thought to be the resistance of insoluble proteins to proteolysis by cellular enzymes. In addition, separation of insoluble recombinant proteins in inclusion bodies is considerably easier than that of soluble proteins. These factors have been the major influences favoring scale-up of high-value proteins using bacterial fermentation for example. Procedures for the purification of the expressed proteins from inclusion bodies are often labour-intensive, time consuming and not cost effective. This kit provides the essential reagents for cell disruption, inclusion body solubilization and purification using spin column chromatography - all optimized to work together thereby simplifying the process and saving a tremendous amount of time and cost.